Research
The Role Of Mitochondrial Mutations In Cancer
What is our research about?
In my lab we study the potential role of mitochondria in cancer formation. The majority of tumors—including cancers of the colon, breast, bladder, head and neck, lung, prostate and ovary—have abnormalities in their metabolism and exhibit somatic (acquired during the lifetime of the individual, not inherited) mutations in the mitochondrial chromosome (mtDNA). Cancer cells are largely glycolytic, deriving their cellular energy, ATP, from glycolysis rather than mitochondrial respiration. Defects in mitochondria potentially promote cancer cell development via three mechanisms: a.) defects in programmed cell death (apoptosis), which is used by normal tissue to prevent the accrual of damaged cells and for which mitochondria are necessary; b.) increased levels of reactive oxygen species which result in DNA damage to cancer genes; or c.) abnormally high activation of cell proliferation signals from the mitogenic factor HIF-1a, which is also caused by increased levels of reactive oxygen species.
Over 65% of cancers examined show mutations in the mitochondrial chromosome (mtDNA), which codes for components of the electron transport chain. Do these mtDNA mutations play a causal role in cancer formation or are they merely a side effect in the abnormal cancer cell microenvironment? To address this question, we characterize somatic mtDNA mutations in cancer.
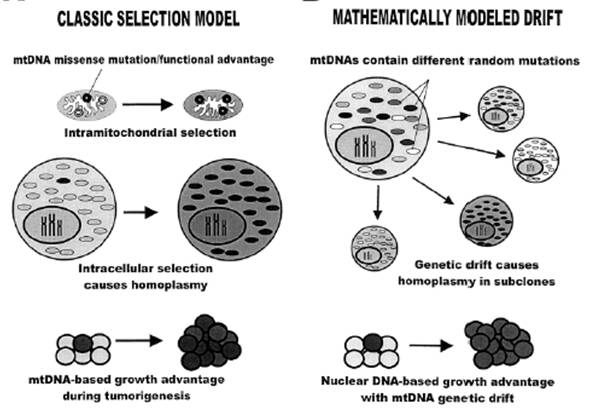
Figure 1 (ABOVE) Are mtDNA mutations in cancer due to chance or are they selected for in the growing tumor? Figure from Jones et al., 2001. Cancer Res 61(4):1299-304.
If mtDNA mutations are only a result of the cancer environment, then homoplasmic somatic mutations detected in a mature tumor are primarily due to drift, under random or neutral forces (Fig 1-right.) If cancer mtDNA mutations confer a cell proliferation advantage, then the observed DNA variation should be due to positive selection.
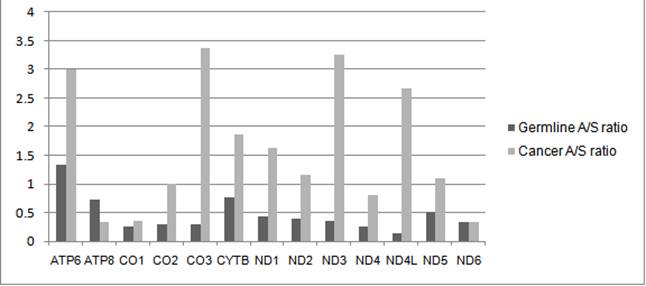
Figure 2 (ABOVE) The rate of amino acid changing mutations in mtDNA is much higher in a cancer tumor than occurs at the whole organism level. Figure from Stafford and Chen-Quin, 2010.
Here we observe the ratio of amino acid mutations to silent mutations (A/S) in cancer tumors vs. the human gene pool in the thirteen genes of the mitochondrial chromosome. It shows excess amino acid mutations in cancer. mtDNA genes ND3, CO3, and ND4L in particular show amino acid changes 9 to 18 times more frequently in cancer than at the germline level. These results suggest that the mitochondrial chromosome has fewer functional constraints within tumor cells and are thus able to accumulate more mutations.









