Research
Projects in Mensa-Wilmot Laboratory
Trypanosoma spp. are eukaryote microorganisms that cause human and veterinary diseases. Nagana in cattle and pigs is caused by Trypanosoma brucei brucei. Surra in horses, camels, llamas, and cats is caused by T. evansi, and Human African trypanosomiasis (HAT) is caused by T. b. gambiense and T. b. rhodesiense.
We have a diversified research portofolio conisisting of four independent projects. In the first two, we are developing two chemical scaffolds into drugs for treatment of human African trypanosomiasis [1-5]). The next pair of projects are basic biology studies employing genetic and small-molecule approaches to understand mechanisms of (a) mitochondrial DNA inheritance [6, 7], and (b) regulation of endocytosis by protein kinases [8, 9].
Below we summarize three of the projects:
-
Mitochondrial Genome Division in the African Trypanosome
The mitochondrial genome of T. brucei is organized as a single network of thousands of catenated circular DNAs associated with proteins (and termed kinetoplast) [10]. Loss of a kinetoplast breaks transmission of the trypanosome from tsetse fly vectors to vertebrates [11-14].
In G1 of a cell cyle T. brucei has a single kinetoplast (K) and one nucleus (N) (i.e., 1K1N) (Fig. 1). Kinetoplast DNA (kDNA) is synthesized in S-phase [15], and trypanosomes with two kinetoplasts per cell (i.e., in 2K1N trypanosomes) are observed in G2 [7, 16]. Thus, a kinetoplast is duplicated before mitosis occurs. Nuclei of 2K1N trypanosomes divide to generate 2K2N cells that yield two 1K1N cells after cytokinesis [7, 13].
Fig. 1 Kinetoplasts division cycle visualized by fluorescence microscopy
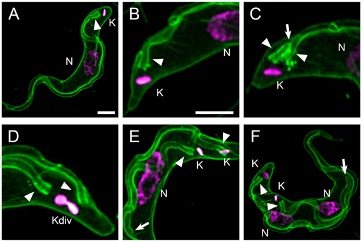 Trypanosomes were incubated with mCLING-488 (green) on ice, fixed, and DNA stained with DAPI (magenta) [17]. (A) Plasma membrane and flagellum stain with mCLING. (B, C) Plasma and flagellar membranes of a 1K1N trypanosome. Fagella are labeled with arrowheads. (D) Kinetoplast, dividing (Kdiv). (E) Divided kinetoplasts (K) in a 2K1N cell. (F) Two kinetoplasts and two nuclei in one cell (2K2N). Arrow points to tip of a growing flagellum. Arrowheads indicate posterior bases of flagella. K = kinetoplast; Kdiv = dividing K; N = nucleus. Scale bar = 2 µm.
Trypanosomes were incubated with mCLING-488 (green) on ice, fixed, and DNA stained with DAPI (magenta) [17]. (A) Plasma membrane and flagellum stain with mCLING. (B, C) Plasma and flagellar membranes of a 1K1N trypanosome. Fagella are labeled with arrowheads. (D) Kinetoplast, dividing (Kdiv). (E) Divided kinetoplasts (K) in a 2K1N cell. (F) Two kinetoplasts and two nuclei in one cell (2K2N). Arrow points to tip of a growing flagellum. Arrowheads indicate posterior bases of flagella. K = kinetoplast; Kdiv = dividing K; N = nucleus. Scale bar = 2 µm.Fig. 2 Electron Microscope Images of Kinetoplasts at Different Stages of Division or Partitioning.
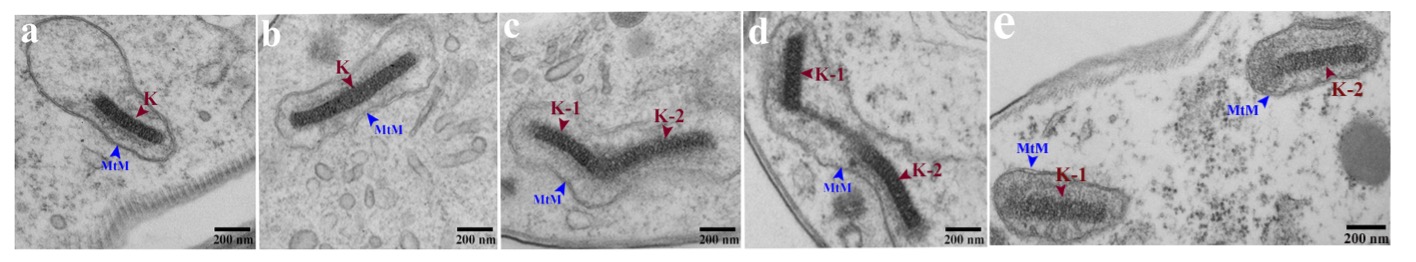 (a) Unit length kinetoplast (K) (450 nm); (b) double-length kinetoplast; (c) scission stage kinetoplast; (d) separating kinetoplasts (K1 and K2); (e) separated kinetoplasts, each surrounded by a mitochondrial membrane.
(a) Unit length kinetoplast (K) (450 nm); (b) double-length kinetoplast; (c) scission stage kinetoplast; (d) separating kinetoplasts (K1 and K2); (e) separated kinetoplasts, each surrounded by a mitochondrial membrane.Although genes associated with inheritance of kinetoplasts have been identified (reviewed in [18]) the mechanism for division (i.e., scission and separation) of kinetoplasts is not known. We have recently documented several proteins that are essential for division of kDNA, that we termed kinetoplast division factors (KDFs) (discussed in [6]). The first KDF studied is casein kinase TbCK1.2. Knockdown of TbCK1.2 yields trypanosomes containing two nuclei but one kinetoplast (1K2N), consistent with the enzymes projected regulatory role in division of kDNA [19]. We are investigating molecular mechanisms by which TbCK1.2 modulates division of kDNA.
-
Lead Drugs for Human African Trypanosomiasis (HAT) Treatment
The first HAT drug in fifty years was approved several months ago. For protozoan parasites, however, the likelihood of resistance developing to any drug in the field is very high. For this reason, it is important that new chemical entities are identified continuously, and developed into drugs, in preparation for trypanosome resistance to drugs in current use.
CBL0137 Cures Human African Trypanosomiasis in a Mouse Model of the Disease
We discovered the carbazole CBL0137 (Fig. 3A) as a new lead against T. brucei. In a murine model of HAT, orally administered CBL0137 cured the disease [3]. In collaborative work with Andrei Purmal (Buffalo BioLabs) and Mike Pollastri (Northeastern University) we are producing new derivatives of CBL0137 that will be optimized using a screening funnel (Fig. 3B) for efficacy, safety, pharmacokinetics, and metabolic properties before designation as drug(s) to treat HAT.Fig. 3 CBL0137 cures HAT in a mouse model
A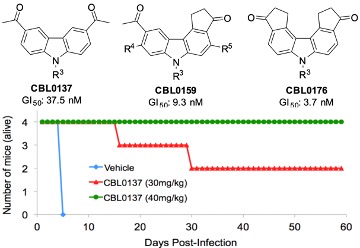 B
B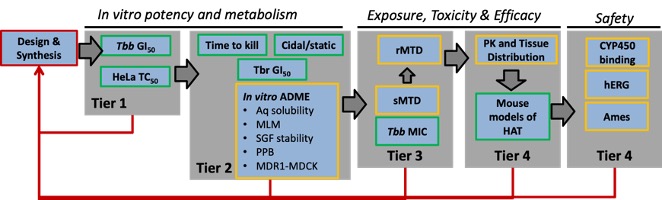
We are studying the molecular pharmacology of CBL0137 in T. brucei. We have identified 10 trypanosome proteins that can form a complex with the drug, as part of an effort to find targets of CBL0137. In mode of action studies, CBL0137 is polypharmacological, like many drugs (reviewed in
[20]); it inhibits mitosis at low concentrations (200 nM) [3], and prevents DNA synthesis in higher amounts (780 nM). -
Endocytosis of Transferrin in a Trypanosome
Iron is essential for proliferation of bloodstream T. brucei in a vertebrate host [21, 22], and it is obtained by receptor-mediated endocytosis of transferrin (Tf), a host protein, that is complexed to the metal [23]. Thus, transferrin is at the interface of trypanosome interaction with a vertebrate host.
Many aspects of endocytosis of Tf are not understood, since most proteins used for endocytosis in human cells are not detected in T. brucei [24-27]. Using gene knock downs and chemical tools, we have documented a role of protein phosphorylation in selective Tf endocytosis [7-9, 28, 29]. We recently identified a pseudokinase TbNRP1 as a regulator of Tf endocytosis. Knockdown of TbNRP1 reduces endocytosis of Tf without inhibiting Haptoglobin-Hemoglobin (HpHb) or BSA (bovine serum albumin) uptake.
Our preliminary work shows that knockdown of TbNRP1 causes de-phosphorylation of proteins, including four protein kinases. Our goals are (i) to discover proteins that act “downstream” of TbNRP1 in a signaling cascade, and (ii) to evaluate other proteins whose phosphorylation was perturbed by knockdown of TbNRP1 as regulators of endocytosis.
The long-term impact of these studies are three-fold. First, we establish that GPI-anchored proteins have different intracellular trafficking fates in T. brucei, in contrast to dogma in the field [30]. Second, we provide functions for a pseudokinase whose roles are currently unknown. Third, we discover new proteins that are important for Tf endocytosis, using TbNRP1 as a genetic and protein tool.
-
Literature Cited
- Bachovchin KA, Sharma A, Bag S, Klug DM, Schneider KM, Singh B, Jalani HB, Buskes MJ, Mehta N, Tanghe S et al: Improvement of Aqueous Solubility of Lapatinib-Derived Analogues: Identification of a Quinolinimine Lead for Human African Trypanosomiasis Drug Development. Journal of medicinal chemistry 2019, 62(2):665-687.
- Woodring JL, Behera R, Sharma A, Wiedeman J, Patel G, Singh B, Guyett P, Amata E, Erath J, Roncal N et al: Series of Alkynyl-Substituted Thienopyrimidines as Inhibitors of Protozoan Parasite Proliferation. ACS Med Chem Lett 2018, 9(10):996-1001.
- Thomas SM, Purmal A, Pollastri M, Mensa-Wilmot K: Discovery of a carbazole-derived lead drug for human African trypanosomiasis. Scientific Reports 2016, 6:32083.
- Patel G, Karver CE, Behera R, Guyett PJ, Sullenberger C, Edwards P, Roncal NE, Mensa-Wilmot K, Pollastri MP: Kinase scaffold repurposing for neglected disease drug discovery: discovery of an efficacious, lapatinib-derived lead compound for trypanosomiasis. J Med Chem 2013, 56(10):3820-3832.
- Katiyar S, Kufareva I, Behera R, Thomas SM, Ogata Y, Pollastri M, Abagyan R, Mensa-Wilmot K: Lapatinib-binding protein kinases in the african trypanosome: identification of cellular targets for kinase-directed chemical scaffolds. PLoS One 2013, 8(2):e56150.
- Mensa-Wilmot K, Hoffman B, Wiedeman J, Sullenberger C, Sharma A: Kinetoplast Division Factors in a Trypanosome. Trends Parasitol 2019, 35(2):119-128.
- Sullenberger C, Pique D, Ogata Y, Mensa-Wilmot K: AEE788 Inhibits Basal Body Assembly and Blocks DNA Replication in the African Trypanosome. Mol Pharmacol 2017, 91(5):482-498.
- Guyett PJ, Xia S, Swinney DC, Pollastri MP, Mensa-Wilmot K: Glycogen Synthase Kinase 3beta Promotes the Endocytosis of Transferrin in the African Trypanosome. ACS Infect Dis 2016, 2(7):518-528.
- Subramanya S, Mensa-Wilmot K: Diacylglycerol-stimulated endocytosis of transferrin in trypanosomatids is dependent on tyrosine kinase activity. PLoS One 2010, 5(1):e8538.
- Jensen RE, Englund PT: Network news: the replication of kinetoplast DNA. Annual review of microbiology 2012, 66:473-491.
- Schnaufer A, Domingo GJ, Stuart K: Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. Int J Parasitol 2002, 32(9):1071-1084.
- Woodward R, Gull K: Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. Journal of cell science 1990, 95 ( Pt 1):49-57.
- Sherwin T, Gull K: The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos Trans R Soc Lond B Biol Sci 1989, 323(1218):573-588.
- Vaughan S, Gull K: Basal body structure and cell cycle-dependent biogenesis in Trypanosoma brucei. Cilia 2015, 5:5.
- Brown SV, Hosking P, Li J, Williams N: ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot Cell 2006, 5(1):45-53.
- Siegel TN, Hekstra DR, Cross GA: Analysis of the Trypanosoma brucei cell cycle by quantitative DAPI imaging. Mol Biochem Parasitol 2008, 160(2):171-174.
- Wiedeman J, Mensa-Wilmot K: A fixable probe for visualizing flagella and plasma membranes of the African trypanosome. PLoS One 2018, 13(5):e0197541.
- Schneider A, Ochsenreiter T: Failure is not an option - mitochondrial genome segregation in trypanosomes. J Cell Sci 2018, 131(18).
- Sullenberger C, Hoffman B, Wiedeman J, Kumar G, Mensa-Wilmot K: Casein kinase TbCK1.2 regulates division of kinetoplast DNA, and movement of basal bodies in the African trypanosome. PLoS One 2021, 16(4):e0249908.
- Mensa-Wilmot K: How Physiological Targets Can Be Distinguished from Drug-binding Proteins. Mol Pharmacol 2021.
- Merschjohann K, Steverding D: In vitro growth inhibition of bloodstream forms of Trypanosoma brucei and Trypanosoma congolense by iron chelators. Kinetoplastid Biol Dis 2006, 5:3.
- Breidbach T, Scory S, Krauth-Siegel RL, Steverding D: Growth inhibition of bloodstream forms of Trypanosoma brucei by the iron chelator deferoxamine. Int J Parasitol 2002, 32(4):473-479.
- Schell D, Evers R, Preis D, Ziegelbauer K, Kiefer H, Lottspeich F, Cornelissen AW, Overath P: A transferrin-binding protein of Trypanosoma brucei is encoded by one of the genes in the variant surface glycoprotein gene expression site. EMBO J 1991, 10(5):1061-1066.
- Grunfelder CG, Engstler M, Weise F, Schwarz H, Stierhof YD, Morgan GW, Field MC, Overath P: Endocytosis of a Glycosylphosphatidylinositol-anchored Protein via Clathrin-coated Vesicles, Sorting by Default in Endosomes, and Exocytosis via RAB11-positive Carriers. Mol Biol Cell 2003, 14(5):2029-2040.
- Allen CL, Goulding D, Field MC: Clathrin-mediated endocytosis is essential in Trypanosoma brucei. Embo J 2003, 22(19):4991-5002.
- Manna PT, Gadelha C, Puttick AE, Field MC: ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis. J Cell Sci 2015, 128(11):2130-2142.
- Garcia-Salcedo JA, Perez-Morga D, Gijon P, Dilbeck V, Pays E, Nolan DP: A differential role for actin during the life cycle of Trypanosoma brucei. Embo J 2004, 23(4):780-789.
- Guyett PJ, Behera R, Ogata Y, Pollastri M, Mensa-Wilmot K: Novel Effects of Lapatinib Revealed in the African Trypanosome by Using Hypothesis-Generating Proteomics and Chemical Biology Strategies. Antimicrob Agents Chemother 2017, 61(2).
- Subramanya S, Hardin CF, Steverding D, Mensa-Wilmot K: Glycosylphosphatidylinositol-specific phospholipase C regulates transferrin endocytosis in the African trypanosome. Biochem J 2009, 417(3):685-694.
- Pal A, Hall BS, Nesbeth DN, Field HI, Field MC: Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J Biol Chem 2002, 277(11):9529-9539.
Learn more about RESEARCH in the College of Science and Mathematics at Kennesaw State University.









